Results
This product was originally formulated for my wife who developed CRPS post hand surgery in 2017. My background includes pain research in pharma and biotech. This experience led me to hypothesize that certain receptors, ion channels, cytokines and enzymes were involved in the CRPS process. Researching the literature for natural products shown to impact those targets allowed for the original formulation. In the beginning of 2024 I was able to connect with other CRPS sufferers who found remarkable relief with the original formula and gave us the opportunity to test other natural products to improve the formula. The current formula is bringing pain levels of 8-10 down to 1-3 with many of those testing it. Below are examples of individuals who have reported back data while testing the product.
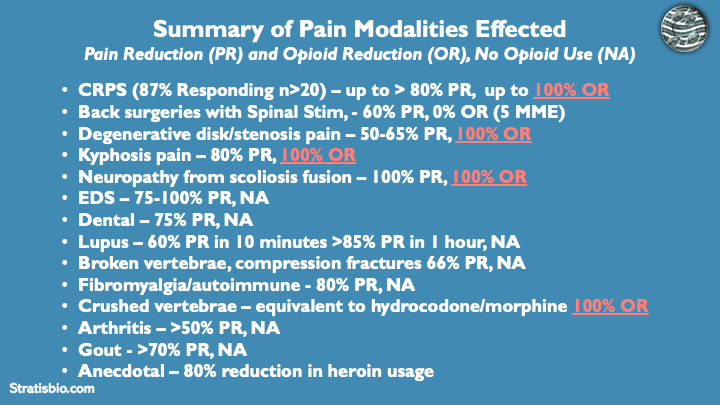
Summary of Efficacy of Product
Revolutionary Relief for CRPS and Chronic Pain
Greater than 85% response rate (n>20), 1 responding marginally, 1 not responding pain wise, 2 not responding at all, and 1 losing sensitivity to the product. 1 reduced MME >80% and 1 can eliminate opioids completely while on the product. This is remarkable as typically less than 30% of CRPS sufferers respond to a treatment.
100% of other chronic pain types tested showed good response for pain.
Importantly:
- 5 of 7 were able to eliminate opioids with no withdrawal
- 1 of 7 reduced >80%
- 1 of 7 did not reduce but was able to not need any rescue doses
Typical cessation of opioids is immediately to within 1-5 days.
Because the formula was so successful in elimination of opioid usage, a heroin addict with high usage rates (multiple doses per day) tried the earliest formula for relief of her cravings. She was able to reduce her heroin usage by 80% when she took the product as an alternative. We have since modified the formula for addiction with early indication it will be a powerful tool against the opioid epidemic. Those data are below.
We are very excited by the Lupus result. This individual is being treated with the newest antibody available and still has pain in the 7-8 level by the end of the day. Seeing 60% pain relief within 10 minutes is truly interesting and we believe early blood analysis of active compound absorption will lead us to the proteins involved in Lupus pain.
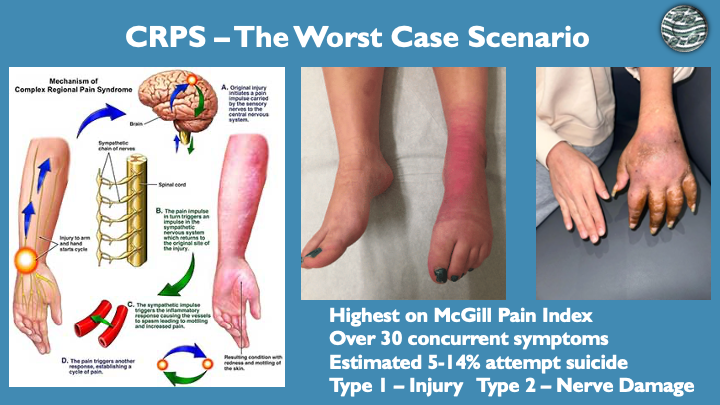
The formula was originally formulated for my wife after she developed CRPS post hand surgery in 2017. CRPS is the most painful disease known to man and is likened to having an amputation without anesthesia, with the pain and burning never subsiding. This is why it is called the suicide disease as it is estimated that over 70% of sufferers go through suicisal idealization and 5-15% attempt suicide at least once.
Stratisbio
Key Facts About CRPS:
-
Approximately 7 people with CRPS attempt suicide daily in the US
-
Only 10-30% of sufferers respond to standard treatments
-
5-14% of CRPS patients attempt suicide at least once
-
Over 70% of sufferers consider suicide seriously (idealization, the final step before an attempt)
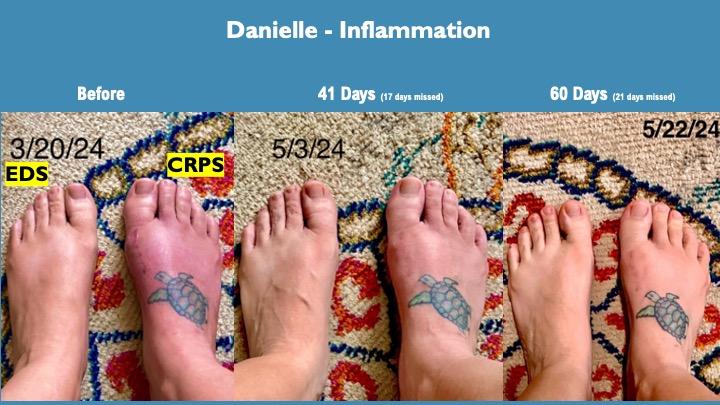
Danielle's Story
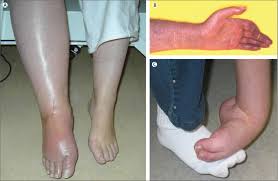
Danielle has EDS, fibromyalgia and CRPS which is consistent with the observation that many CRPS patients have underlying pain disorders before developing CRPS.
Her left foot is swollen and painful from constant subluxations (dislocations) due to EDS. Her right foot is classic CRPS inflammation and swelling.
The reduction in inflammation over testing in both her feet were an early indicator of the strong anti-inflammatory impact of this formulation, especially considering standard anti-inflammatories have no impact. This inflammation impact is very consistent across testing of CRPS and other chronic pain modalities.
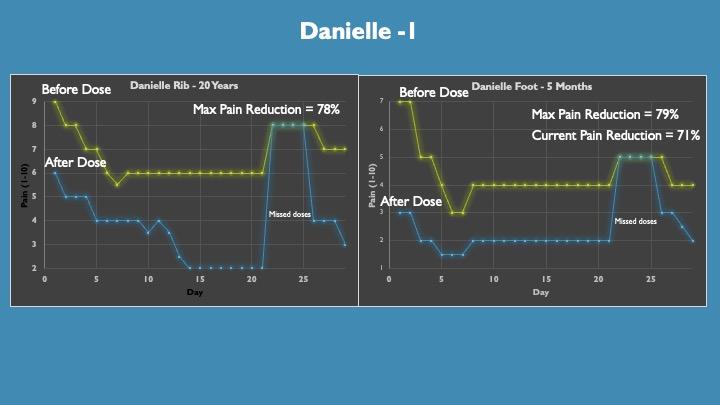
This is a single dose a day protocol as we were trying variations of the formulation and wanted to allow for 24-hour washout.
The data are represented as the pain level before the dose was taken in the am (yellow line) and 1 hour post dose (blue line). Half max pain relief was achieved at 30 minutes and max at 1 hour, thus one hour data are plotted. Pain relief typically lasted 4 to 8 hours with a slow return of pain.
Danielle has two sites of CRPS, her ribs from a car accident in 2004 and her foot from surgery in January. Danielle has not responded to prescription medicines and was opioid treatment resistant. She is currently doing PT.
Her 1st dose gave 33% relief in her ribs and 57% relief in her foot consistent with typical treatment responses of older verses newer CRPS sites.
Surprisingly, we saw baseline pain decrease over time with concomitant treatment pain reduced which is most likely attributed to the reduction of inflammation over time.
Danielle typically sees a reduction of pain from an 8 to a 1 with the current formulation.
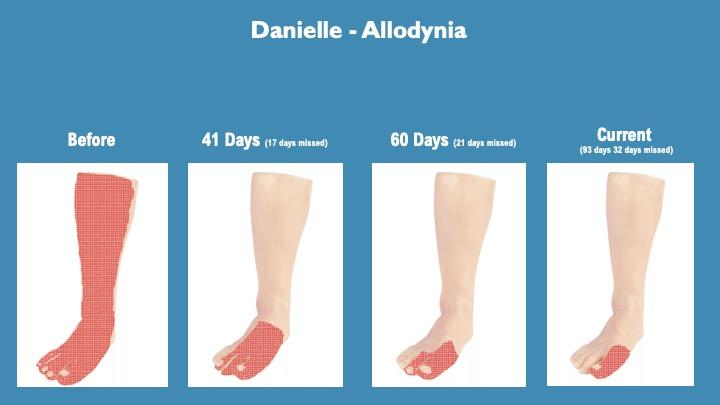
Allodynia is hypersensitivity to touch, giving an abnormal pain response. This is represented by the red shading on her left leg.
Danielle’s allodynia was very severe, sensitive to the slightest touch and air currents. Indeed, pressing a finger into the area of allodynia on her left leg felt like a cigarette being extinguished on the leg.
During testing the area of her allodynia has significantly reduced.
The region originally encompassed the top of the foot, shin, 1/3 of the area into the calf on either side up to the knee, represented by the red fill.
Currently her allodynia covers the top of the hallux (big toe) up to the end of the first metatarsal.
These graphics were made with self-reporting from Danielle
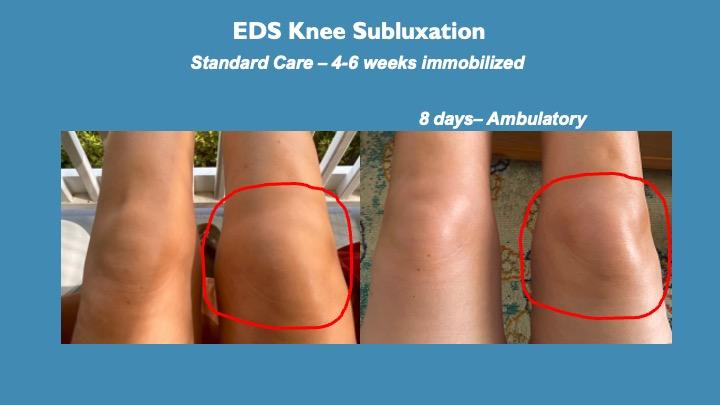
Danielle recently subluxated (dislocated) her right knee due to her EDS. Her typical protocol for this injury is 4-6 weeks braced and immobilized to reduce swelling and recover functionality.
She achieved equivalent results with the product in 8 days without reduction in activity, bracing and K Taping for outdoor work only. These results highlight the anti-inflammatory properties of the formulation as well as pain relief in that Danielle maintained her normal activities
CRPS comes with up to 40 other symptoms such as severe burning sensation, hair loss, immobility, gastroparesis, feeling of disconnection from the affected limb and others. In our responding patients we typically see a >50% reduction of concomitant symptoms of CRPS.
Daniel saw many of her symptoms decrease or cease including symptoms from her EDS. It’s interesting to see hair and nail growth return, suggesting major systemic changes with treatment.
We have found consistently that although the product reduces flare frequency, all subjects still flare due to weather or overactivity. In a flare, pain relief typically lasts 3-4 hours and subsequent dosing may reduce pain further from the initial dose.
The asthma reduction in my opinion is not due to an asthma target interaction but due to overall inflammation reduction.
Quality of life has improved significantly for all responders.

Caryn's Story
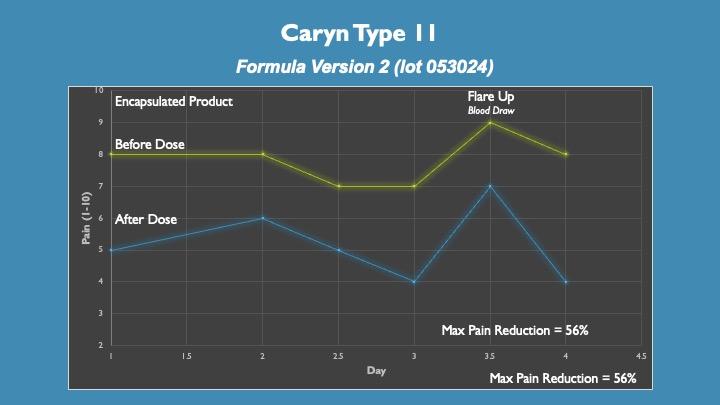
Note: This is encapsulated product
Caryn has Type II CRPS and it has spread whole body. She suffers from gastroparesis and could not drink the liquid dose without vomiting. She encapsulated the product in 00 gelatin capsules taking approximately 10-15 capsules.
Although she sees significant pain relief it doesn’t appear as effective as liquid dosing. This repeats a prior testing of encapsulation. Her gastroparesis is resolving and soon she will attempt to switch to liquid dosing. We have one other Type II sufferer whose gastroparesis did resolve.
On day 3.5 Caryn had a blood draw which triggered a flare up. Flare ups are common with CRPS. We typically see slightly less efficacy during a flare up and a shortened time of relief after dose (i.e. 4 hours from non-flare up relief of 6-8 hours). Importantly she recovered quickly the following day.
We are investigating larger capsules, increasing the dosing and adding other constituents to increase efficacy of the capsules.
Lastly this is an earlier version of the formulation. The current formula has proven more efficacious.
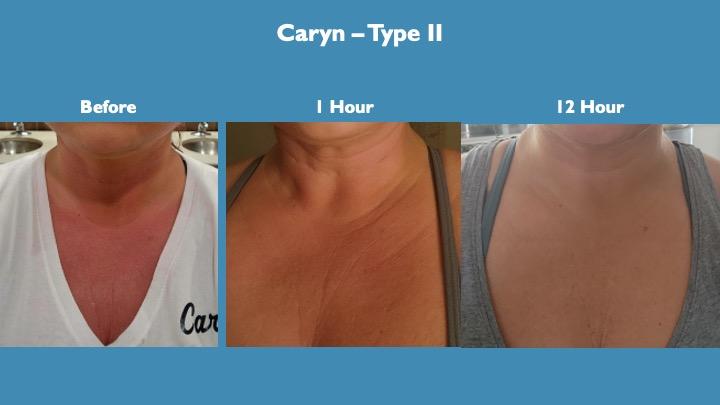
Caryn’s chest regularly shows inflammation and redness which may be erythema. Unfortunately for Caryn she was called out of town for a family medical emergency. In that month away the extreme stress of the situation triggered a flare up with the redness and inflammation. She was able to test product upon returning home and the results were truly remarkable with significant inflammation relief within one hour of taking a dose and complete relief overnight.
The rapidness of this result, complete resolution in 9-12 hours with a significant change at 1 hour was totally unexpected and speaks to the strong anti-inflammatory effect of the product
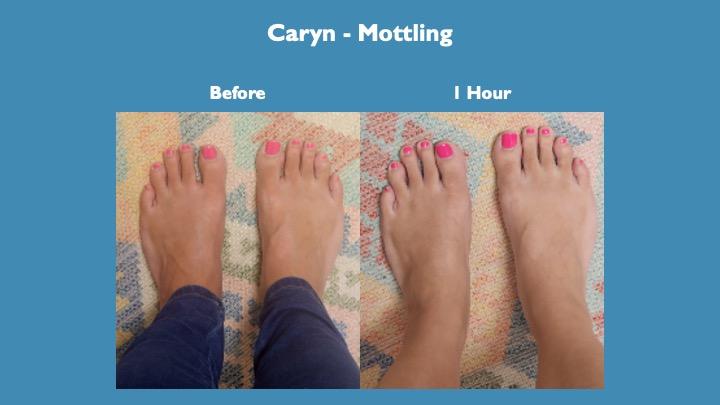
Mottling of the skin in her left foot (slight purple hue) is resolved within one hour of taking the product.
Future testing is planned to include temperature changes of the skin

Caryn suffered from a good number of concomitant symptoms, especially as her CRPS has impacted her whole body.
We saw reduction in the number of symptoms post dosing and it looked like they were being resolved until she had the flare from the blood draw and they mostly returned (Day 3.5 data).
Fortunately, by day four she had 60% of her symptoms resolved. This type of response is consistent among the responders although a flare can bring them back.
We are continually hearing from the CRPS sufferers how they can return to normal activities that we typically take for granted. Personally, this has been one of the most rewarding parts of this project.

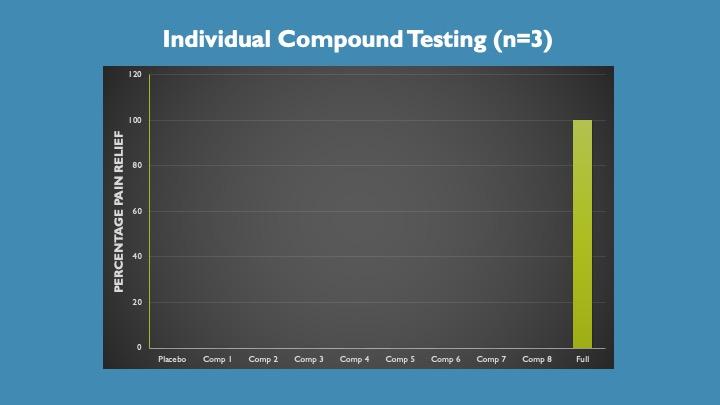
Pain is a difficult condition for drug development because of the placebo effect. Indeed, many pharma’s have abandoned pain drug development because of the difficulty showing significance over placebo.
Here we tested, with three individuals, a placebo, the placebo mixed with the individual components at their typical dose level and then the whole product.
The placebo and each individual component had no effect on pain relative to the full product, displayed as percentage of total pain relief in the testing.
The synergistic effect is remarkable and speaks to the approach of targeting pain signaling in multiple pathways.
Note, there are no error bars as there was no variation. Further, there are an additional 2 components in the current formulation not represented here.
An individual with severe stenosis (narrowing of nerve canals from the spine) and degenerative disk did a direct comparison of this product to their Vicodin protocol.
They found the results of the product equivalent to the Vicodin protocol and could be completely substituted the product.

Most every one of us understand the urgency of the opioid crisis in the US. About 2.5 million people are affected by opioid use disorder (addiction). About 75% of them started with prescription opioid use, perhaps post and injury or surgery. Tragically, there are 220 opioid deaths daily in the US with many being in the prime of their lives. We believe this pain-relieving formula offers a non-addictive substitute for opioid prescriptions and will significantly reduce the number of new individuals becoming addicted to opiates. Currently, 3,000 individuals misuse opioids for the first time EVERY day.
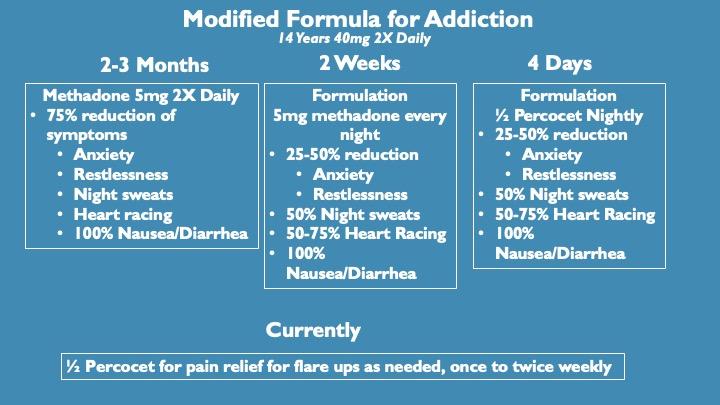
We modified the pain formula specifically targeting receptors, ion channels and other proteins involved in addiction and tested it with one of the most difficult to quit opioids available, Methadone. Because of its long half-life, it is not as immediately addictive as heroin for example, but once dependent it is much harder to quit than heroin as withdrawal symptoms can last for weeks.
This individual was taking high doses of methadone, originally prescribed off label for pain. Throughout that time, she tried many times to stop methadone unsuccessfully. The protocol of reduction in methadone dose followed by integration of the modified formula led to diminished withdrawal symptoms and complete relief of methadone dependence in just over 2 weeks.
We’ve seen minimum adverse effects with the product. Indigestion is more common, about 30% of individuals. We have yet to try acid reducers or slippery elm (similar to Sucralfate but not limited to ulcers or erosions). This will be done if indigestion persists.
The burning in the mouth and lips of one individual is most likely due to a necessary component of the mixture, an extract of black pepper.
We had one individual who fell asleep within an hour of taking the product and couldn’t report pain changes. She was very sleep deprived from CRPS disturbing her sleep and we hypothesized that with pain relief she could finally sleep. With continued use the lethargy resolved and she received significant pain relief. We have not seen other cases of lethargy.
We are developing a formula for night time which relieves pain and assists with sleep.

For a more in depth review of multiple examples please contact Jef Pfohl at jpfohl_stratisbio@proton.me
Handling pain without addictive prescription drugs and elimination of opioid use.
Join us in our fight against CRPS, chronic pain, and opioid addiction. Together, we can make a difference.

