Welcome to Stratisbio
A possible breakthrough in Chronic Pain, CRPS management and Opioid Dependency.
Handling pain without addictive prescription drugs and elimination of opioid use.
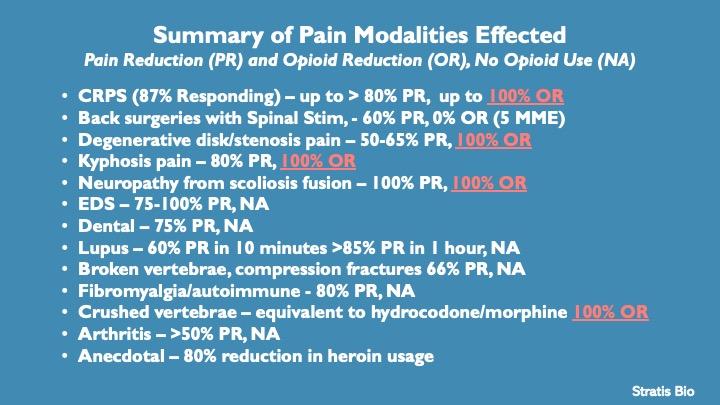
Summary of individual experiences with product
Revolutionary Relief for CRPS and Chronic Pain
The product was developed for CRPS (Chronic Regional Pain Syndrome) a rare neurological disease described below. Remarkably, 87% of sufferers (20 of 23) responded with significant, often life changing, pain relief. This is notable in that typically the response rate to individual treatments is around 10-30% which is why this disease is called the suicide disease. Many have gone from pain levels of 8-9 down to a 1-3. Further, many of these individuals have already exhausted other treatments with no effects.
With regards to other chronic pain disease sufferer’s results, we have a 100% response rate so far with 70% being able to eliminate the use of opioids in 1-7 days. Others are able to reduce opioid use by >80%
Because the formula was so successful in elimination of opioid usage, a heroin addict with high usage rates (multiple doses per day) tried the earliest formula for relief of her cravings. She was able to reduce her heroin usage by 80% when she took the product as an alternative. We have since modified the formula for addiction with early indications showing it will be a powerful tool against the opioid epidemic.
The product was originally formulated for my wife after she developed CRPS post hand surgery in 2017. CRPS is the most painful disease known to man and is likened to having an amputation without anesthesia, with the pain and burning never subsiding. This is why it is called the suicide disease as it is estimated that over 70% of sufferers go through suicidal idealization and 5-15% attempt suicide at least once. This translates to 7 people attempting suicide every day in the US!
Stratisbio
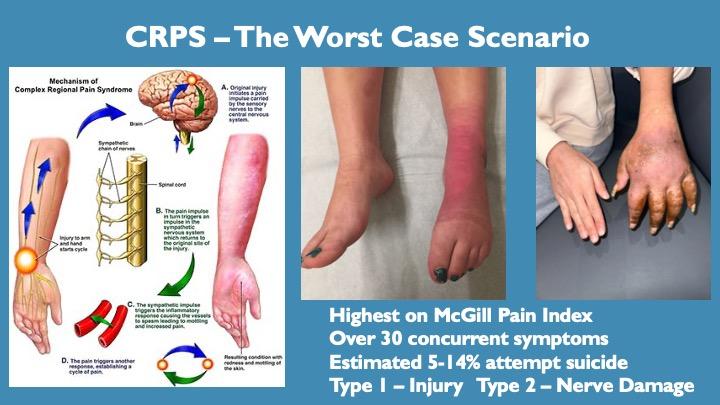
Key Facts About CRPS:
-
Most painful disease known to man
-
Only 10-30% of sufferers respond to standard treatments
-
5-14% of CRPS patients attempt suicide at least once
-
Over 70% of sufferers consider suicide seriously (idealization, the final step before an attempt)
-
Approximately 7 people with CRPS attempt suicide daily in the US

Success Stories
Case Studies
Danielle's Stories
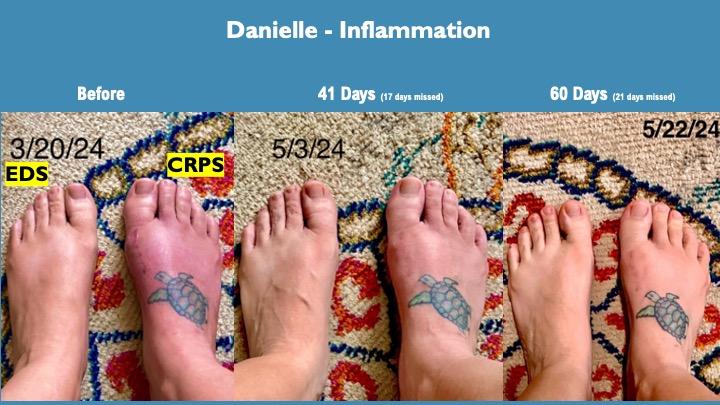
Danielle has EDS, fibromyalgia and CRPS which is consistent with the observation that many CRPS patients have underlying pain disorders before developing CRPS.
Her left foot is swollen and painful from constant subluxations (dislocations) due to her EDS. Her right foot is severely inflamed, painful and swollen from her CRPS.
The reduction in inflammation over testing in both her feet were an early indicator of the strong anti-inflammatory impact of this formulation, especially considering standard anti-inflammatories had minimal impact for her. This inflammation impact is very consistent across testing of CRPS and other chronic pain modalities.
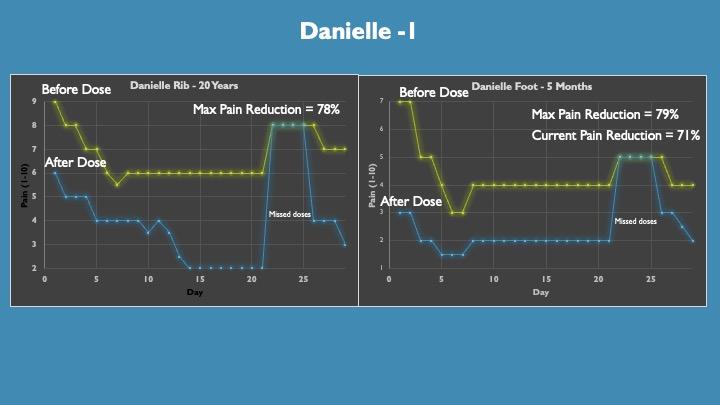
This is a single dose a day protocol as we were trying variations of the formulation and wanted to allow for 24-hour washout.
The data are represented as the pain level before the dose was taken in the am (yellow line) and 1 hour post dose (blue line). Half max pain relief was achieved at 30 minutes and max at 1 hour, thus one hour data are plotted. Pain relief typically lasted 4 to 8 hours with a slow return of pain.
Danielle has two sites of CRPS, her ribs from a car accident in 2004 and her foot from surgery in January. Danielle has not responded to prescription medicines and was opioid treatment resistant. She is currently doing PT.
Her 1st dose gave 33% relief in her ribs and 57% relief in her foot consistent with typical treatment responses of older verses newer CRPS sites.
Surprisingly, we saw baseline pain decrease over time with concomitant treatment pain reduced which is most likely attributed to the reduction of inflammation over time.
Danielle typically sees a reduction of pain from an 8 to a 1 with the current formulation.
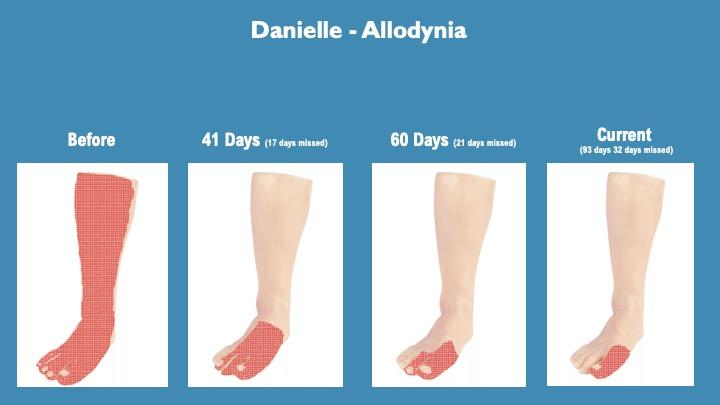
Allodynia is hypersensitivity to touch, giving an abnormal pain response. This is represented by the red shading on her left leg.
Danielle’s allodynia was very severe, sensitive to the slightest touch and air currents. Indeed, pressing a finger into the area of allodynia on her left leg felt like a cigarette being extinguished on the leg.
During testing the area of her allodynia has significantly reduced.
The region originally encompassed the top of the foot, shin, 1/3 of the area into the calf on either side up to the knee, represented by the red fill.
Currently her allodynia covers the top of the hallux (big toe) up to the end of the first metatarsal.
These graphics were made with self-reporting from Danielle
Open for Appointments
Lorem ipsum dolor sit amet, consecte adipiscing elit
We are continually hearing from the CRPS sufferers how they can return to normal activities that we typically take for granted. Personally, this has been one of the most rewarding parts of this project.

Most every one of us understand the urgency of the opioid crisis in the US. About 2.5 million people are affected by opioid use disorder (addiction). About 75% of them started with prescription opioid use, perhaps post and injury or surgery. Tragically, there are 220 opioid deaths daily in the US with many being in the prime of their lives. We believe this pain-relieving formula offers a non-addictive substitute for opioid prescriptions and will significantly reduce the number of new individuals becoming addicted to opiates. Currently, 3,000 individuals misuse opioids for the first time EVERY day.
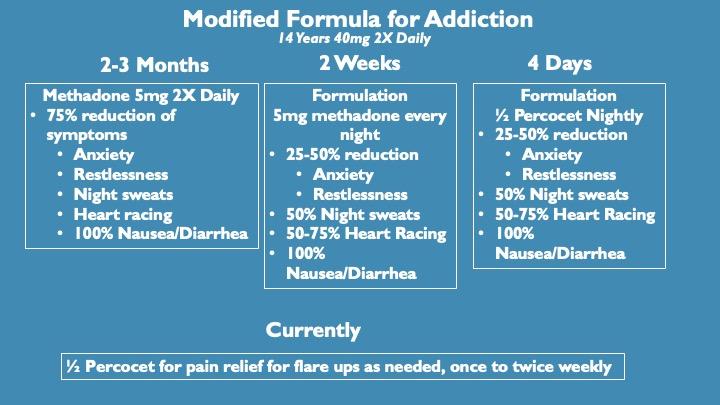
We modified the pain formula specifically targeting receptors, ion channels and other proteins involved in addiction and tested it with one of the most difficult to quit opioids available, Methadone. Because of its long half-life, it is not as immediately addictive as heroin for example, but once dependent it is much harder to quit than heroin as withdrawal symptoms can last for weeks.
This individual was taking high doses of methadone, originally prescribed off label for pain, for. Throughout that time, she tried many times to stop methadone unsuccessfully. The protocol of reduction in methadone dose followed by integration of the modified formula led to diminished withdrawal symptoms and complete relief of methadone dependence in just over 2 weeks.
Our Mission: Accessible Relief for All
We aim to:
- Bring this life-changing formula to market
- Provide relief to CRPS patients as quickly as possible
- Expand to the broader chronic pain community
- Address the opioid crisis
- Build nonprofit to help those who can’t afford to purchase the product
The cost of bringing this type of formulation to market with clinical data is well over $1 million. This covers the cost of manufacturing and all of the requirements for FDA approval.
Based on the results, we strongly feel that this formulation can help change countless lives because of its versatility.

Currently we will be releasing the product as a supplement as we generate data for final FDA approval.
Handling pain without addictive prescription drugs and elimination of opioid use.
Join us in our fight against CRPS, chronic pain, and opioid addiction. Together, we can make a difference.
